Electrosurgical RF Medical Devices and Development
Electrosurgical Devices, RF Microneedling, and OEM Support
RF Kinetics designs and delivers electrosurgical and RF-based medical devices, supporting both proprietary product lines and engineering-led product development programs.
Surgical RF Devices and Development Expertise
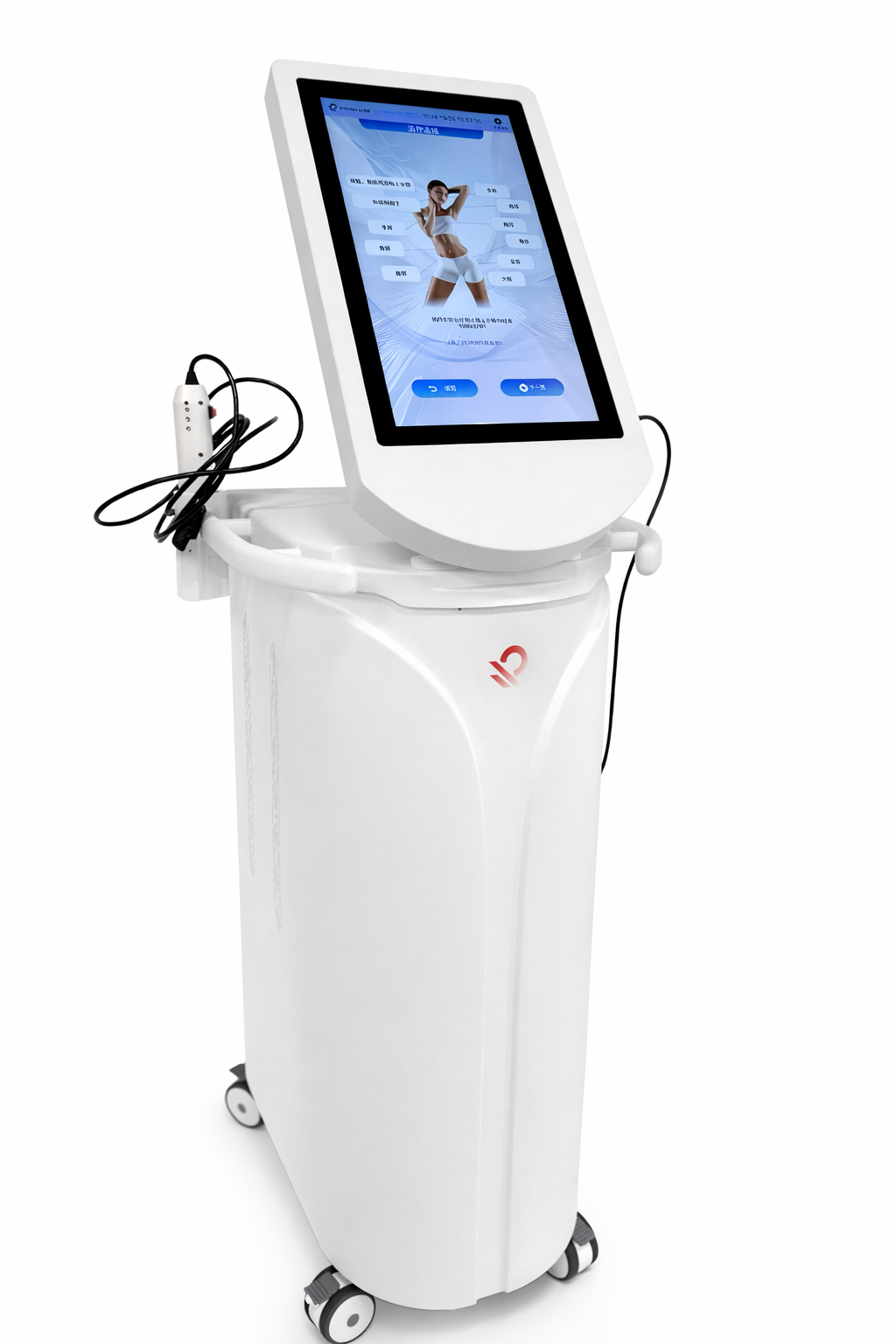
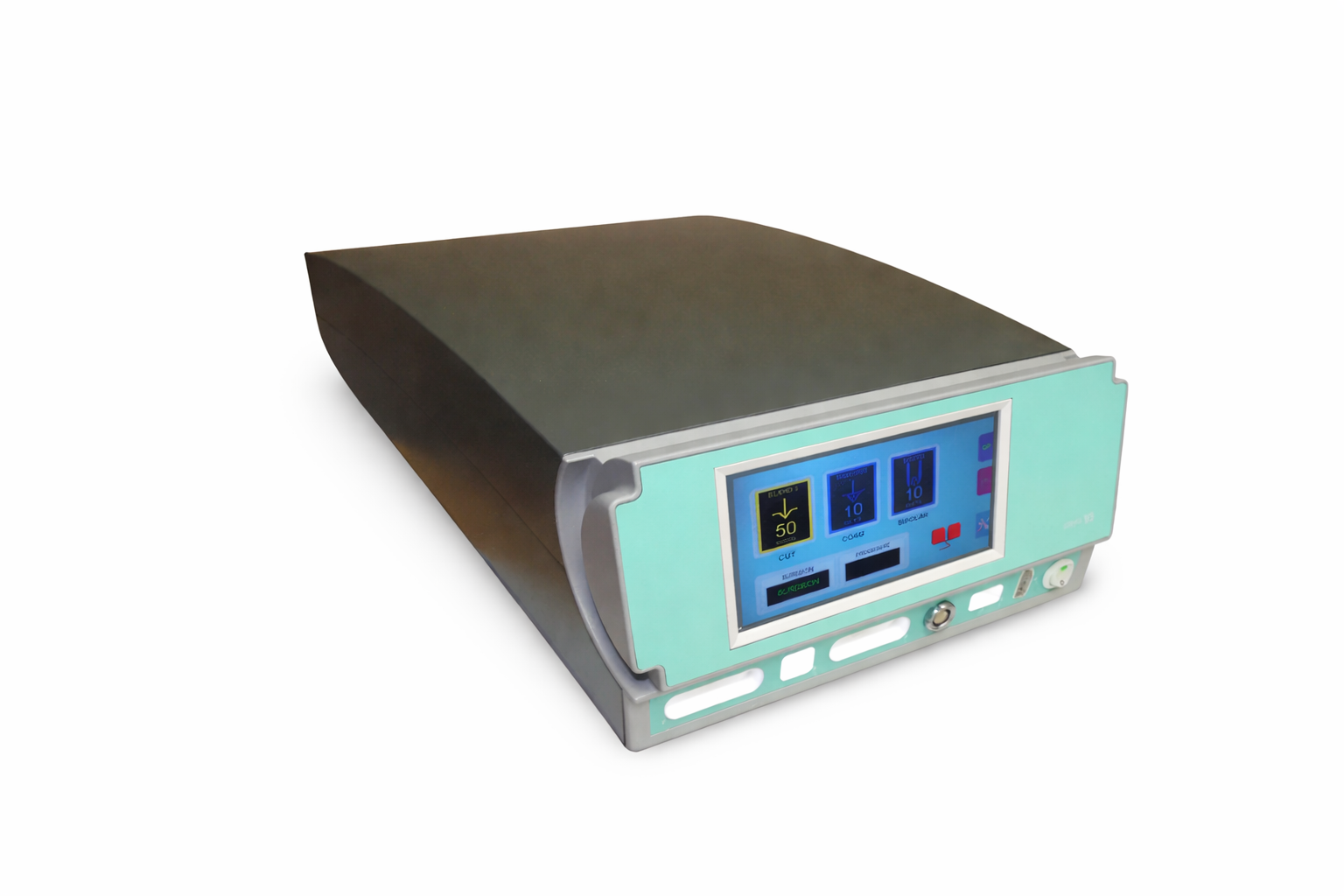
RF Kinetics develops and supplies commercial radiofrequency-based surgical devices used in a range of clinical and procedural environments. Our product portfolio includes electrosurgical electrodes, RF generators, vessel sealing systems, and RF microneedling platforms designed for reliability, repeatability, and scalable production.
Alongside our commercial products, RF Kinetics provides development and manufacturing support for OEM and strategic partners. Our capabilities encompass device design, engineering, prototyping, process development, and regulated manufacturing, enabling partners to advance new surgical technologies from concept through commercial production.
Electrosurgical and RF Medical Device Products
Our product portfolio includes electrosurgical devices and RF-based technologies designed for clinical use.
Electrosurgical Electrodes
Single-use and reusable electrodes designed for precision, durability, and consistent RF performance.
Electrosurgical Pencils
Handheld RF instruments engineered for ergonomic control, reliability, and procedural efficiency in the operating room.
RF Microneedling Systems
RF platforms designed for controlled dermal energy delivery in aesthetic and clinical treatment applications.
Vessel Sealing Systems
Integrated RF generators and instruments developed for controlled tissue sealing, hemostasis, and reproducible surgical outcomes.
Medical Device Product Development and OEM Support
RF Kinetics provides end-to-end medical device development and manufacturing for OEM partners and clinical innovators.
In addition to our commercial product portfolio, we work collaboratively with partners to design, engineer, and produce radiofrequency-based surgical devices and related instruments.
Our capabilities span early-stage concept development, mechanical and electrical engineering, prototyping, process development, verification support, and scalable manufacturing. Programs are executed within controlled processes appropriate for regulated medical devices, with an emphasis on performance consistency, manufacturability, and long-term reliability.
Core Capabilities
Our internal capabilities support programs from early development through commercial production:
Medical device concept development and system architecture
RF-based device engineering and prototyping
Process development and design for manufacturability
Verification support and transition to production
Regulated manufacturing for commercial medical devices
Quality control activities at the RF Kinetics facility, performed in a clean room.
Regulatory Compliance
Engineering and manufacturing activities are conducted under ISO 13485–compliant quality systems. Devices are designed and manufactured in alignment with applicable international standards, including ISO and IEC requirements, to support regulatory compliance for FDA and CE Mark pathways.